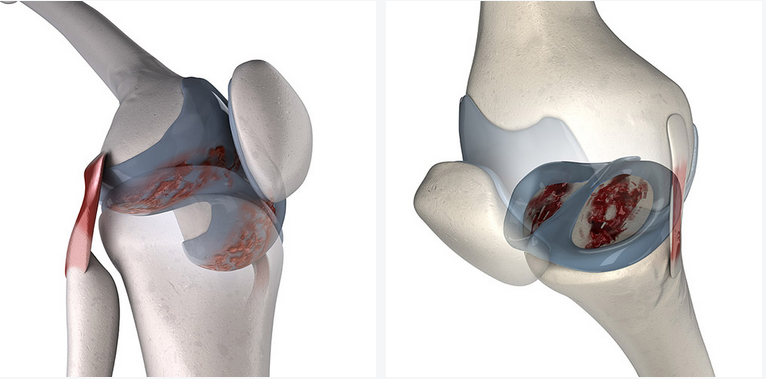
Articular Cartilage Research
Many people suffering from severe arthritis and major cartilage damage are told they will need artificial joint replacement surgery. Artificial joint replacement can be successful in eliminating joint pain, but it prohibits impact sports and leaves patients with very limited treatment options when unsuccessful. The researchers at the Stone Research Foundation seek minimally invasive, biologic ways to regenerate cartilage, in order to delay or avoid the need for artificial joint replacement.
Femoral Condyle Cartilage
Tibial Plateau Cartilage
Articular Cartilage History
Articular cartilage paste grafting started in 1991 when Dr. Kevin Stone observed a presentation by Lanny Johnson showing several cases of bone grafting of damaged areas of the knee joint where the joint healed in pretty well. He also observed that when the intercondylar notch of the knee was widened during ACL reconstruction, the notch grew nicely with what looked like shiny, healthy articular cartilage.
He felt that if the articular cartilage could grow in this spot, then maybe if transferred to another area, it could grow as well. He also thought that if cells interacted with a matrix of articular cartilage and bone, the matrix would stimulate the cells to form cartilage instead of bone. Finally, he thought that the primary cells in the bone marrow responsible for healing might benefit the cartilage and bone repair.
Grow It Inside, Not Outside the Knee
From these ideas, Dr. Stone developed the articular cartilage paste grafting technique whereby bone and cartilage is harvested from the intercondylar notch, taken out of the joint, smashed into a paste, and finally impacted into a defect that has been prepared using a super microfracture or morselization technique. As part of an IRB-approved study, 70 patients undergoing the articular cartilage paste grafting technique returned for a second-look arthroscopy and biopsy of their cartilage-repaired tissue. The biopsies were processed for histological evaluation and sent for independent review by Donald Speer. Dr. Speer reported that 1/3 of the specimens had a nearly normal histologic articular cartilage appearance, 1/3 appeared to be a mixture of fibrous and fibrocartilage, and 1/3 appeared to be purely fibrous tissue. These results were published in 2006.
Alternative Cartilage Repairs
The field of articular cartilage repair expanded greatly with the introduction of other techniques, including ACI, OATs, and mosaicplasty procedures. Each of the other procedures has had significant downfalls, including that they were not indicated in the setting of arthritis with all requiring harvesting tissue from one part of the joint and transferring it to another and creating an injury in another part of the joint, or requiring an open knee procedure. The ACI, OATs, and Mosaicplasty procedures were also significantly more expensive than articular cartilage paste grafting, since paste grafting used reusable trephines from a normal operating room.
Microfracture became the most widely used technique to repair defects in the knee. However, microfracture led to fibrous repair tissue and failure in many athletes after a few years. Microfracture was not indicated for arthritis. A definitive study published in 2012 comparing microfracture to paste graft demonstrated:
Articular cartilage paste grafting, which uses the patients’ own stem cells, cartilage, and bone to repair arthritic defects in the knee joint, produces superior tissue when compared to microfracture.
The tissue is superior because it has stem cells in it, and the DNA in those cells makes more normal-looking smooth cartilage repair tissue.
There appears to be a synergistic interaction of the stem cells with the cartilage matrix and the cells of the cartilage (chondrocytes) that exceeds what might have been expected from cells alone.
A custom-designed set of instruments was produced by DePuy for Dr. Stone in the early 1990’s which greatly facilitated the ease of the procedure being performed. Since that time, Dr. Stone has performed hundreds of articular cartilage paste graft procedures with long-term follow-up. The procedure has also been useful to salvage failed microfracture and OATs and failed OCD repairs. The Stone Research Foundation recently collected a series of patients with very large lesions from failed osteochondritis dissecans repairs, which were successfully repaired with articular cartilage paste grafts. This procedure has allowed some patients to return to sports such as triathlons and running marathons and appears to last up to 20 years.
Surgical Technique Video: Articular Cartilage Paste Graft
CURRENT STUDIES
Articular Cartilage Paste Graft in Large Animal Models Study
We are improving the paste graft procedure and making it more widely acceptable to surgeons. We are developing and testing a slowly absorbable hydrogel additive in collaboration with Dr. Anthony Ratcliffe of Synthasome and Dr. David Frisbie at Colorado State University. The hydrogel paste graft combination will improve adherence of the paste and augment cell migration and expansion, thereby speeding up the repair and regeneration of chondral defects from sports injuries and arthritis.
Hydrogel and Articular Cartilage Paste Graft Study
Hydrogels have been considered a promising material for the delivery of cells and growth factors. They can serve as a temporary and artificial extracellular matrix, on which cells can adhere and proliferate. Understanding the composition and cellular viability of the articular cartilage paste graft matrix from human subjects once mixed with hydrogels can clarify the potential clinical performance of the paste graft and may lead to advances in hybrid composites with cohesive agents and potentially biological factors to enhance cartilage healing.
Articular Cartilage Paste Graft in Humans Clinical Trial
Articular cartilage paste graft can be used as a strategy to treat early-onset osteoarthritis and in turn prevent or delay chronic OA. We aim to conduct a clinical trial to promote the safety, efficacy, and ease of the articular cartilage paste graft technique to orthopaedic surgeons and professional organizations around the world focused on treating joint cartilage lesions and osteoarthritis.
Injections of Growth Factors to Improve Osteoarthritis Study
Injection of growth factors into an osteoarthritic joint can activate and recruit local support cells to the diseased area and result in a positive, anabolic, and prolonged effect. The primary purpose of this study is to examine whether certain growth factors can reduce inflammation and pain in joints and accelerate healing following osteoarthritis onset, injury, or surgery.
Articular Cartilage Paste Graft for Severe Osteochondral Lesions of the Knee: A 10 - 20 Year Follow-Up Study
We aim to report 10-20 year clinical outcomes of the articular cartilage paste graft procedure for severe knee cartilage damage, as measured by duration of procedure-induced benefit and subjective outcome scores.
Lab-On-A-Chip And Nanoparticles For Promoters Of Cell Growth Study
Biomaterials demonstrate great promise for use as tools in a wide range of biomedical applications. In collaboration with Dr. DeCoster at Louisiana Tech University, we will be testing the effect of nanoparticles on a 3D Lab-On-A-Chip model. Dr. DeCoster and his lab have discovered a novel, copper-containing and amino-acid-based high aspect ratio structure material. The discovery of this material has opened up the possibility of creating functionalized biomaterials with growth factors aimed at alleviating osteoarthritis.
ONGOING AND PREVIOUS STUDIES
Articular Cartilage Thin Shell Graft Study
2007 - PRESENT
Glucosamine and Athletic Performance
2007 - 2009
Salvage and Repair of Osteochondritis Dissecans with Articular Cartilage Paste Grafting
2002 - PRESENT
Articular Cartilage Paste Grafting to Traumatic and Arthritic Knee Joint Defects
1991 - PRESENT
COLLABORATING INSTITUTIONS:
Pregenerate Inc. (Dr. Julie Rosser)
Snythasome Inc. (Dr. Anthony Ratcliffe)
Louisiana Tech University (Dr. Mark Decoster)
Feinstein Medical Research Inst. (Dr. Dan Grande)
Northeastern (Dr. Jeff Ruberti)
Colorado State University (Dr. David Frisbie)
SELECTED PUBLICATIONS
Osteochondral Autograft Plugs versus Paste Graft: Ex Vivo Morselization Increases Chondral Matrix Production. Daniel Grande, Todd Goldstein, Thomas J. Turek, Susan Hennessy, Ann W. Walgenbach, Le Hanh Dung Do, David Greene, Kevin R. Stone.
Study Link: https://journals.sagepub.com/doi/10.1177/1947603520916552
ABSTRACT
Objective
Patients undergoing articular cartilage paste grafting have been shown in studies to have significant improvement in pain and function in long-term follow-ups. We hypothesized that ex vivo impacting of osteochondral autografts results in higher chondrocyte matrix production versus intact osteochondral autograft plugs.
Design
This institutional review board–approved study characterizes the effects of impacting osteochondral plugs harvested from the intercondylar notch of 16 patients into a paste, leaving one graft intact as a control. Cell viability/proliferation, collagen type I/II, SOX-9, and aggrecan gene expression via qRT-PCR (quantitative reverse transcription-polymerase chain reaction) were analyzed at 24 and 48 hours. Matrix production and cell morphology were evaluated using histology.
Results
Paste samples from patients (mean age 39.7) with moderate (19%) to severe (81%) cartilage lesions displayed 34% and 80% greater cell proliferation compared to plugs at 24 and 48 hours post processing, respectively (P = 0.015 and P = 0.021). qRT-PCR analysis yielded a significant (P = 0.000) increase of aggrecan, SOX-9, collagen type I and II at both 24 and 48 hours. Histological examination displayed cell division throughout paste samples, with accumulation of aggrecan around multiple chondrocyte lacunae.
Conclusions
Paste graft preparation resulted in increased mobility of chondrocytes by matrix disruption without loss of cell viability. The impaction procedure stimulated chondrocyte proliferation resulting in a cellular response to reestablish native extracellular matrix. Analysis of gene expression supports a regenerative process of cartilage tissue formation and contradicts long-held beliefs that impaction trauma leads to immediate cell death. This mechanism of action translates into clinical benefit for patients with moderate to severe cartilage damage.
Osteochondral grafting for failed knee osteochondritis dissecans repairs. Stone KR, Pelsis JR, Crues JV, Walgenbach AW, Turek TJ. Knee (2014), http://dx.doi.org/10.1016/j.knee.2014.09.003.
ABSTRACT
Background: Revision of failed surgical treatments of osteochondritis dissecans (OCD) lesions remains a challenge without an obvious solution. The aim of this study was to evaluate seven consecutive patients undergoing osteochondral grafting of a failed OCD repair. Methods: The mean time from surgery to the latest evaluation was 7.0 years. IKDC, WOMAC, Tegner and MRI studies were collected both preoperatively and during follow-up. Evaluation of the graft was assessed using the magnetic resonance observation of cartilage repair tissue (MOCART) grading system. Results: Over the course of the study period, 5 patients required additional surgery with a study median of 1 additional surgery (range, 0–3). At most recent follow-up, there was significant improvement from preoperative values in median IKDC (p= 0.004), WOMAC (p= 0.030), and Tegner (p= 0.012). Complete cartilage fill and adjacent tissue integration of the paste graft were observed byMRI evaluation in 5 of the 7 (71.4%) patients. Definitive correlation between clinical outcomes and MRI scores was not observed. Conclusions: This study shows promising results of osteochondral grafting as a viable option for the revision failed OCD lesion repairs, however more patients are needed to fully support its efficacy in these challenging failed revision cases.
Articular Cartilage Paste Grafting to Full-Thickness Articular Cartilage Knee Joint Lesions: A 2- to 12-Year Follow-up. Stone KR, Walgenbach AW, Freyer A, Turek TJ, Speer DP. Arthroscopy: The Journal of Arthroscopic and Related Surgery, Vol 22, No 3 (March), 2006: pp 291-299.
ABSTRACT
PURPOSE: To prospectively assess clinical outcomes and regeneration of osteoarthritic cartilage lesions treated with an articular cartilage paste grafting technique. TYPE OF STUDY: Prospective, longitudinal case series. METHODS: We treated 125 patients (136 procedures; 34% female, 66% male; mean age, 46 years; range, 17 to 73 years) with an Outerbridge classification of grade IV lesions with an articular cartilage paste graft. Clinical data were recorded 2 to 12 years from surgery, with 20 of 145 patients lost to follow-up over 12 years (13.7%). Clinical outcomes were captured annually with validated Western Ontario and McMaster Universities Arthritis Index (WOMAC), International Knee Documentation Committee (IKDC), and Tegner subjective questionnaires. Regenerated cartilage biopsy specimens were obtained at second-look arthroscopy from 66 patients and evaluated as to quality and quantity of defect fill by a blinded, independent histopathology reviewer. RESULTS: Preoperative versus postoperative validated pain, functioning, and activity measures improved significantly (P< .001). Clinically, 18 of the 125 patients were considered failures (14.4%), with 10 patients undergoing subsequent joint arthroplasty and 8 who reported worse pain after surgery. Regional histologic variation occurred. Forty-two of 66 biopsy specimens (63.6%) showed strong and consistent evidence of replacement of their articular surface, and 18 of 66 biopsy specimens (27.3%) showed development of areas of cartilage. CONCLUSIONS: Paste grafting is a low-cost, 1-stage arthroscopic treatment for patients with Outerbridge classification grade IV arthritic chondral lesions. The procedure offers excellent, long-lasting, pain relief, restored functioning, and possibility of tissue regeneration for patients with painful chondral lesions in both arthritic and traumatically injured knees. LEVEL OF EVIDENCE: Level IV, case series.
Articular Cartilage Paste Grafting: Use in Primary Lesions and Failed Chondroplasty. Stone KR, Walgenbach AW, Keller LE, Freyer A. Basic Science, Clinical Repair and Reconstruction of Articular Cartilage Defects: Current Status and Prospects, 2006.
ABSTRACT
PURPOSE: To prospectively assess clinical outcomes and regeneration of osteoarthritic cartilage lesions treated with an articular cartilage paste grafting technique. TYPE OF STUDY: Prospective, longitudinal case series. METHODS: We treated 125 patients (136 procedures; 34% female, 66% male; mean age, 46 years; range, 17 to 73 years) with an Outerbridge classification of grade IV lesions with an articular cartilage paste graft. Clinical data were recorded 2 to 12 years from surgery, with 20 of 145 patients lost to follow-up over 12 years (13.7%). Clinical outcomes were captured annually with validated Western Ontario and McMaster Universities Arthritis Index (WOMAC), International Knee Documentation Committee (IKDC), and Tegner subjective questionnaires. Regenerated cartilage biopsy specimens were obtained at second-look arthroscopy from 66 patients and evaluated as to quality and quantity of defect fill by a blinded, independent histopathology reviewer. RESULTS: Preoperative versus postoperative validated pain, functioning, and activity measures improved significantly (P< .001). Clinically, 18 of the 125 patients were considered failures (14.4%), with 10 patients undergoing subsequent joint arthroplasty and 8 who reported worse pain after surgery. Regional histologic variation occurred. Forty-two of 66 biopsy specimens (63.6%) showed strong and consistent evidence of replacement of their articular surface, and 18 of 66 biopsy specimens (27.3%) showed development of areas of cartilage. CONCLUSIONS: Paste grafting is a low-cost, 1-stage arthroscopic treatment for patients with Outerbridge classification grade IV arthritic chondral lesions. The procedure offers excellent, long-lasting, pain relief, restored functioning, and possibility of tissue regeneration for patients with painful chondral lesions in both arthritic and traumatically injured knees. LEVEL OF EVIDENCE: Level IV, case series.
Salvage of Failed Knee Chondroplasty Using Articular Cartilage Paste Grafting. Stone KR, Walgenbach AW, Keller LE, Smetana B. Postsurgical Orthopedic Sports Rehabilitation - Knee and Shoulder, Manske RC, 2006.
Articular Cartilage Paste Grafting. Stone, KR, Walgenbach, AW. Surgical Techniques for the Knee, Cushner, FD, Scott, WN, Scuderi, GR, 2005.
Articular Cartilage Repair—The Paste Graft Technique. Stone KR. Surgery of the Knee-Third Edition, Insall JN, Scott WN, 2001.
New Techniques for Cartilage Repair and Replacement. Stone KR, Mullin MJ. Knee Ligament Rehabilitation, Ellenbecker TS, June 2000.
Articular Cartilage Paste Grafting: Surgical Techniques and Initial Results. Stone KR, Walgenbach AW. Journal of Sports Traumatology and Related Research, Vol. 20, No. 2, 1998.
Surgical Technique and Results for Articular Cartilage Transplantation to Traumatic and Arthritic Defects in the Knee Joint. Stone KR, Walgenbach AW. Operative Techniques in Orthopaedics, Vol. 7, No. 4, pg. 305-311, October 1997.
PATENTS
"Cartilage Enhancing Food Supplements and Methods of Preparing the Same." US Patent 6,432,929. Issued August 13, 2002.
"Food Supplement Containing A Cartilage Supplement." US Patent 6,391,864. Issued May 21, 2002.
"Method and Paste for Articular Cartilage Transplantation." US Patent # 6,110,209. Issued August 29, 2000.
"Articular Cartilage Xenographs." US Patent # 6,049,025. Issued April 11, 2000.
"Method and Paste for Articular Cartilage Transplantation." US Patent # 5, 964,805. Issued October 12, 1999.
"Articular Cartilage Surface Shaping Apparatus and Method." US Patent # 5,964,752. Issued October 12, 1999.
"Articular Cartilage Xenografts." US Patent # 5,944,755. Issued August 31, 1999.
"Articular Cartilage Heterografts." US Patent # 5,922,027. Issued July 13, 1999.
"Articular Cartilage Transplant Instrument Set." US Patent # 5,921,987. Issued July 13, 1999.
"Articular Cartilage Heterografts." US Patent # 5,782,915. Issued July 21, 1998.
"Prosthetic Articular Cartilage." US Patent # 5,624,463. Issued April 29, 1997.
"Prosthetic Articular Cartilage." US Patent # 5,306,311. Issued April 26, 1994.
"Apparatus and Method for Measuring Range of Motion of an Articulated Joint." US Patent # 5,253,655. Issued October 19, 1993.