Meniscus Cartilage Research
Meniscus tissues are vital to the healthy functioning of the knee joint. Missing or damaged meniscus tissue can lead to the development of osteoarthritis as the unprotected femur and tibia grind together. By replacing all or a portion of the meniscus with donor cartilage, the patient can regain the natural “shock absorber” in the knee and experience many additional years of activity, even in the presence of arthritis.
MENISCUS CARTILAGE HISTORY
The search for something soft, strong and low friction
Dr. Stone injured his own knee playing soccer at Harvard. The surgeon removed this meniscus. Dr. Stone's quest to find a replacment started when he was out for a run with his mentor Dr. Richard Steadman who suggested that if he could find a replacement he would make a significant contribution to orthopaedics. Stone spent the next few years as an orthopedic surgery resident collecting and reading research on the meniscus cartilage at the Countway Library at Harvard. He gave lectures at Thermedics and Dupont and talked with their scientists searching for artificial materials that might replace the role of the meniscus in the knee. However, since the knee goes through 2 – 3 million cycles per year and the cartilage covering the end of the bones is up to five times as slick as ice-on-ice, there is no material known to man that is soft enough, strong enough, and with a low enough friction to go into the knee joint between these surfaces and not damage healthy articular cartilage.
A shift, a discovery and a step towards meniscus regeneration
In 1986, while doing a research fellowship at the Hospital for Special Surgery, he shifted from replacing the meniscus to a concept of regrowing the meniscus. He turned to a collagen scaffold developed for hemostasis and a crosslinking technology by Ioannis Yannas, PhD, a professor of mechanical and biological engineering at MIT used for skin grafting and decided that since the meniscus (and the whole body) is made of collagen, this could be the best material to use for a regeneration template. Dr. Stone designed a collagen scaffold cross-linked with glycosaminoglycans for the repair of the meniscus. He contracted with Shu-Tung Li, PhD, at American BioMaterials Company to build a collagen scaffold according to a custom design by Dr. Stone loaded with glycosaminoglycans in a similar manner to the meniscus as a possible meniscus cartilage regeneration template.
Tests and awards
Dr. Stone traveled to South Lake Tahoe, California for a clinical knee fellowship with Dr. Richard Steadman. During that time, Dr. Stone wrote a grant to the Letterman Institute for research to fund animal trials of the collagen scaffold. He collaborated with Bill Rodkey, Phil Bowman, Richard Weber, and LuAnn McDermot to conduct a series of studies in tissue culture and animals demonstrating the efficacy of the collagen scaffold for regrowing meniscus cartilage as well as the intervertebral discs for goats. This research and subsequent publications was awarded the Albert Trillat Young Investigators award from the International Society of the Knee in 1989 and the Cabaud Research Award for meniscal regeneration using co-polymeric collagen scaffolds by the American Society of Orthopedics Sports Medicine in 1990.
Surgical Technique
Medial and Lateral Meniscus Transplant
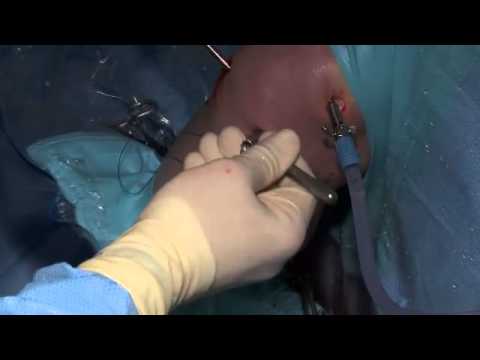
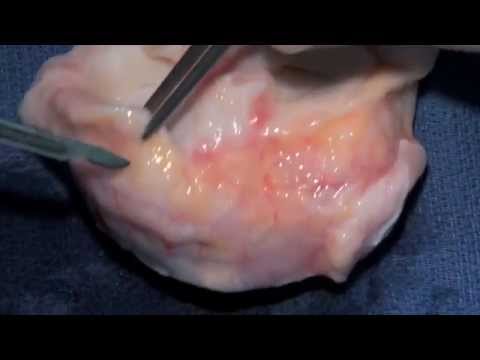
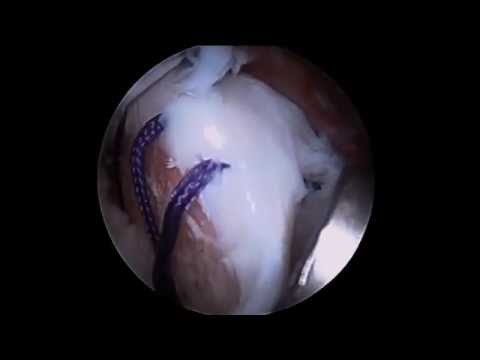
CURRENT STUDIES
Meniscal Allograft Transplantation Outcomes Study
We aim to report clinical outcomes of the meniscal allograft transplantation procedure as measured by duration of procedure-induced benefit and subjective outcome scores.
New Collagen Meniscus Scaffold for Meniscus Regeneration
Meniscus repairs are often not performed due to the complex tearing of the meniscus tissue. In most cases, the torn meniscus is removed which can lead to the development of osteoarthritis. Meniscus regeneration with a collagen implant at the time of the initial surgery may prevent the later onset of osteoarthritis. A stronger collagen structure that supports meniscus regeneration in the knee would have an immediate and substantial impact in orthopaedics and on the quality of life of patients who would otherwise progress to OA. Our long-term objective is to develop a natural meniscus repair device capable of replacing native meniscus tissue yet requires minimal host remodeling.
Meniscus Allograft Transplantations: An Analysis Of Revision Cases And Correlation To Long-Term Clinical Outcomes
We aim to understand the reasons for graft failure that were treated by revision meniscus allograft transplantation and to examine the long-term clinical outcomes of these same patients.
ONGOING AND PREVIOUS STUDIES
Meniscus Sizing Study
2004 - 2005
Meniscal Allograft Replacement Study
1997 - PRESENT
Collagen Meniscus Implant Study
1993 - 2010
SELECTED PUBLICATIONS
Meniscus transplantation in an active population with moderate to severe cartilage damage. Stone, KR, Pelsis, JR, Surrette, ST, Walgenbach, AW, & Turek, TJ (2014). Knee Surg Sports Traumatol Arthrosc.
ABSTRACT
PURPOSE: The purpose of this study was to evaluate the efficacy of meniscus allograft transplantation in an active patient population with moderate to severe cartilage damage and the procedure's ability to allow sports participation postoperatively. METHODS: Forty-nine patients with moderate to severe cartilage damage who underwent meniscus allograft transplantation were included in this study; those with symptoms related to articular cartilage damage also underwent articular cartilage repair. Kaplan-Meier (KM) survival estimate, potential hazards to survival, and subjective clinical outcomes were analyzed. For KM survival, failure was defined as progression to knee arthroplasty, surgical removal of the meniscus transplant without revision, a self-reported follow-up pain level that was more than preoperative level, or constant moderate pain with no relief from non-operative treatment. RESULTS: The mean follow-up time was 8.6 ± 4.2 years. The mean age at surgery was 45.3 ± 12.9 years. Meniscus transplantation was performed in 37 medial cases and 12 lateral cases. There were 41 patients with Outerbridge Grade IV and 8 with Grade III. Thirty-six (73.5 %) patients were able to participate in sporting activities postoperatively. Eleven (22.4 %) meniscus transplants failed at an average of 5.2 ± 4.4 years. The KM mean estimated survival time was 12.6 ± 0.7 years. No tested risks were found to affect sports participation or procedure success. CONCLUSIONS: Meniscus transplantation is a viable surgical option for patients with severe cartilage damage and missing or irreparable menisci to provide significant improvements in pain and function levels in the medium to long term with the majority of patients achieving their goal of participation in sporting activities. These results indicate that symptomatic patients may be able to participate in sports activities for an average of 12.6 years following meniscus transplantation. LEVEL OF EVIDENCE: Case series, Level IV.
Long-term survival of concurrent meniscus allograft transplantation and articular cartilage repair: A Prospective Two- To 12-Year Follow-Up Report. J Bone Joint Surg Br 92-B(7): 941-948. Stone K.R., Adelson W.S., Pelsis J.R., Walgenbach A.W., Turek T.J. 2010.
ABSTRACT
We describe 119 meniscal allograft transplantations performed concurrently with articular cartilage repair in 115 patients with severe articular cartilage damage. In all, 53 (46.1%) of the patients were over the age of 50 at the time of surgery. The mean follow-up was for 5.8 years (2 months to 12.3 years), with 25 procedures (20.1%) failing at a mean of 4.6 years (2 months to 10.4 years). Of these, 18 progressed to knee replacement at a mean of 5.1 years (1.3 to 10.4). The Kaplan-Meier estimated mean survival time for the whole series was 9.9 years (sd 0.4). Cox's proportional hazards model was used to assess the effect of covariates on survival, with age at the time of surgery (p = 0.026) and number of previous operations (p = 0.006) found to be significant. The survival of the transplant was not affected by gender, the severity of cartilage damage, axial alignment, the degree of narrowing of the joint space or medial versus lateral allograft transplantation. Patients experienced significant improvements at all periods of follow-up in subjective outcome measures of pain, activity and function (all p-values < 0.05), with the exception of the seven-year Tegner index score (p = 0.076).
Meniscus reconstruction: the new field of rebuilding meniscus cartilage. Stone KR, Pelsis JR, Adelson, WS, Walgenbach, AW. Knee Surgery, Arthroscopy, Sport Traumatology, 7(3): 9 – 18, 2010.
Meniscal Sizing Based on Gender, Height, and Weight. Stone KR, Freyer A, Turek T, Walgenbach AW. Arthroscopy: The Journal of Arthroscopic and Related Surgery. May 2007:23(5):503-8.
Lessons Learned From Our First 100 Meniscus Allograft Transplants in Arthritic Knees. Stone KR, Walgenbach AW, Freyer A. Musculoskeletal Tissue Regeneration: Biological Materials and Methods, Humana Press, In press.
Meniscus Allograft Survival in Patients with Moderate to Severe Unicompartmental Arthritis: A 2- to 7-Year Follow-up. Stone KR, Walgenbach AW, Turek TJ, Freyer A, Hill M. Arthroscopy: The Journal of Arthroscopic and Related Surgery, Vol 22, No 5 (May), 2006: pp 469-478.
Meniscal Allografting: The Three-Tunnel Technique. Stone KR, Walgenbach AW. Arthroscopy: The Journal of Arthroscopic and Related Surgery, April 2003.
Meniscus Regeneration by Collagen Meniscus Scaffolding. Stone KR. Surgery of the Knee-Third Edition, Insall JN, Scott WN, 2001.
New Techniques for Cartilage Repair and Replacement. Stone KR, Mullin MJ. Knee Ligament Rehabilitation, Ellenbecker TS, June 2000.
Current and Future Directions for Meniscus Repair and Replacement. Stone KR. Clinical Orthopaedics, 367:S273-280, October 1999.
Meniscus Replacement. Stone KR. Clinics in Sports Medicine, Vol. 15, No 3, pg. 557-571, July 1996.
PATENTS
"Substantially Non-Immunogenic Injectable Collagen." US Patent 7,064,187 B2. Issued June 20, 2006.
"Substantially Non-Immunogenic Injectable Collagen." US Patent Application Publication. US 2004/0192603 A1. Issued September 30, 2004.
"Meniscal Heterografts." US Patent # 6,093,204. Issued July 25, 2000.
"Meniscal Xenografts." US Patent # 6,046,379. Issued April 4, 2000.
"Meniscal Augmentation Device." US Patent # 6,042,610. Issued March 28, 2000.
"Osteotomy Wedge Device, Kit and Methods for Realignment of a Varus Angulated Knee." US Patent # 6,008,433. Issued December 28, 1999.
"Meniscal Xenografts." US Patent # 5,984,858. Issued November 16, 1999.
"Substantially Native Meniscal Cartilage Heterografts." US Patent # 5,913,900. Issued June 22, 1999.
"Technique for Osteocartilaginous Transplantation in a Mammalian Joint." US Patent # 5,881,733. Issued March 16, 1999.
"Meniscal Heterografts." US Patent # 5,865,849. Issued February 2, 1999.
"Suture Anchor Assembly." US Patent # 5,824,011. Issued October 20, 1998.
"Meniscal Augmentation Device." US Patent # 5,735,903. Issued April 7, 1998.
"Meniscal Augmentation Device." US Patent # 5,681,353. Issued October 28, 1997.
"Prosthetic Meniscus." US Patent # 5,158,574. Issued October 27, 1992.
"Prosthetic Meniscus." US Patent # 5,116,374. Issued May 26, 1992.
"Prosthetic Meniscus." US Patent # 5,007,934. Issued April 16, 1991.
"Prosthetic Meniscus." US Patent # 4,880,429. Issued November 14, 1989

